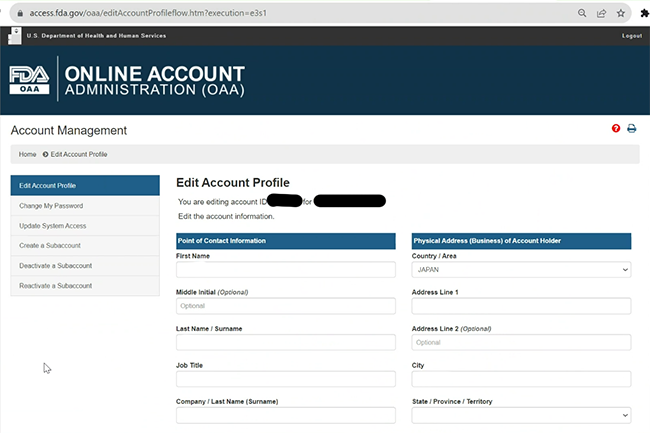
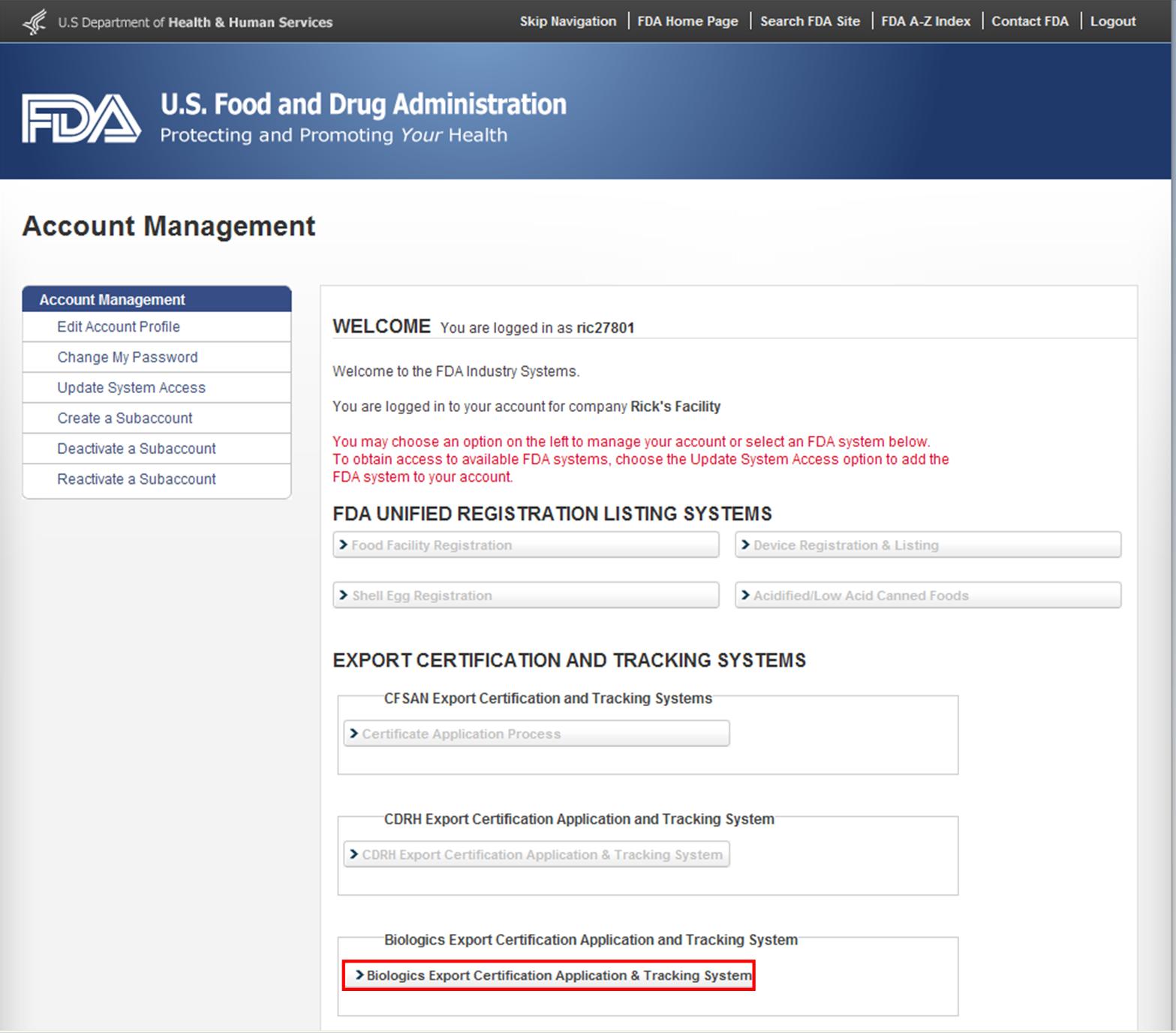
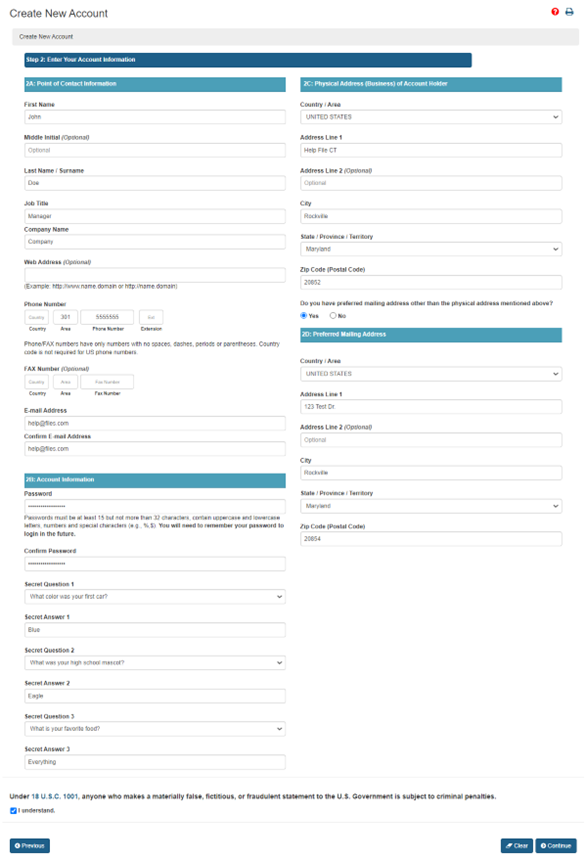
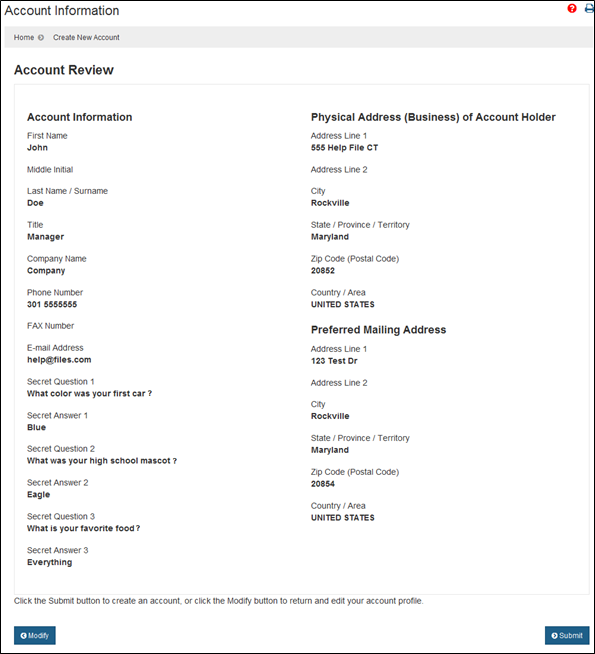
Navigating Regulatory Wasters: A Comparative Dive into FDA Audits vs EU Audits in the Medical Device Industry
28 avril 2023 : Microbiome Post - MaaT Pharma Announces U.S. FDA Lifts Clinical Hold on Phase 3 Investigational New Drug Application for MaaT013 in Patients with Acute Graft-versus-Host Disease (English only) - MaaT Pharma

Smoore Tech Invited to Address FDA Industry Meeting - Smoore International (06969) We are the world's leading atomization technology solution provider

Veolia won the "first gold"! What made the first FDA certification in the China's plastic recycling industry so special? | Veolia China