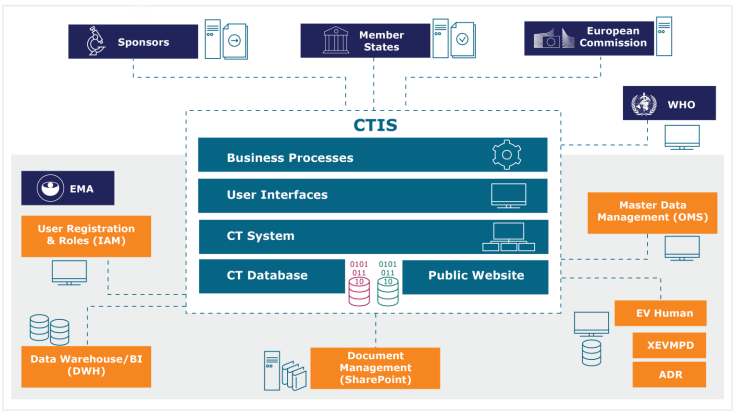
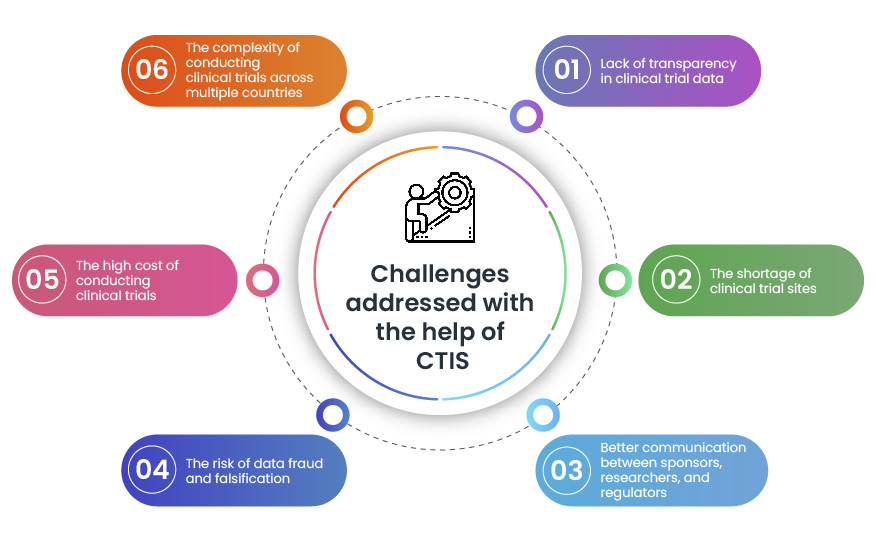
In the current system of clinical trials results dissemination, data... | Download Scientific Diagram

ANSM Agence nationale de sécurité du médicament et des produits de santé sur LinkedIn : Use of Clinical Trials Information System becomes mandatory for new…